The recent decision by Covis Pharma to voluntarily withdraw Makena, the only FDA-approved drug for the prevention of preterm birth, has sparked a robust discourse within the healthcare community. Initially endorsed in 2011 under the FDA’s accelerated approval process, Makena was considered a beacon of hope for at-risk pregnant individuals. The drug, a synthetic form of progesterone, was thought to significantly reduce preterm births, especially given its initial promising results from a 2003 study which suggested a 33% efficacy in preventing such early deliveries.
However, the expectation placed on Makena has been met with skepticism and opposition, particularly following a larger clinical trial conducted in 2020, which indicated that the drug failed to demonstrate any tangible benefits. This development has raised questions about the FDA’s accelerated approval process, which allows medications to enter the market based on preliminary evidence rather than comprehensive clinical trials. Critics argue that this regulatory framework can compromise patient safety and efficacy, a concern that is particularly poignant in scenarios involving maternal and child health.
In response to the 2020 study’s findings, Covis posited that the research was fundamentally flawed, asserting that it examined a lower-risk population compared to the earlier 2003 trial. The company suggested that this discrepancy hindered the study’s ability to yield conclusive evidence regarding the drug’s effectiveness. This assertion points to a troubling dichotomy within clinical research: the complexity of replicating earlier success in different demographic and clinical environments. The implications of such discrepancies are vast, particularly when interventions like Makena are scrutinized amid increasing rates of preterm births in the United States.
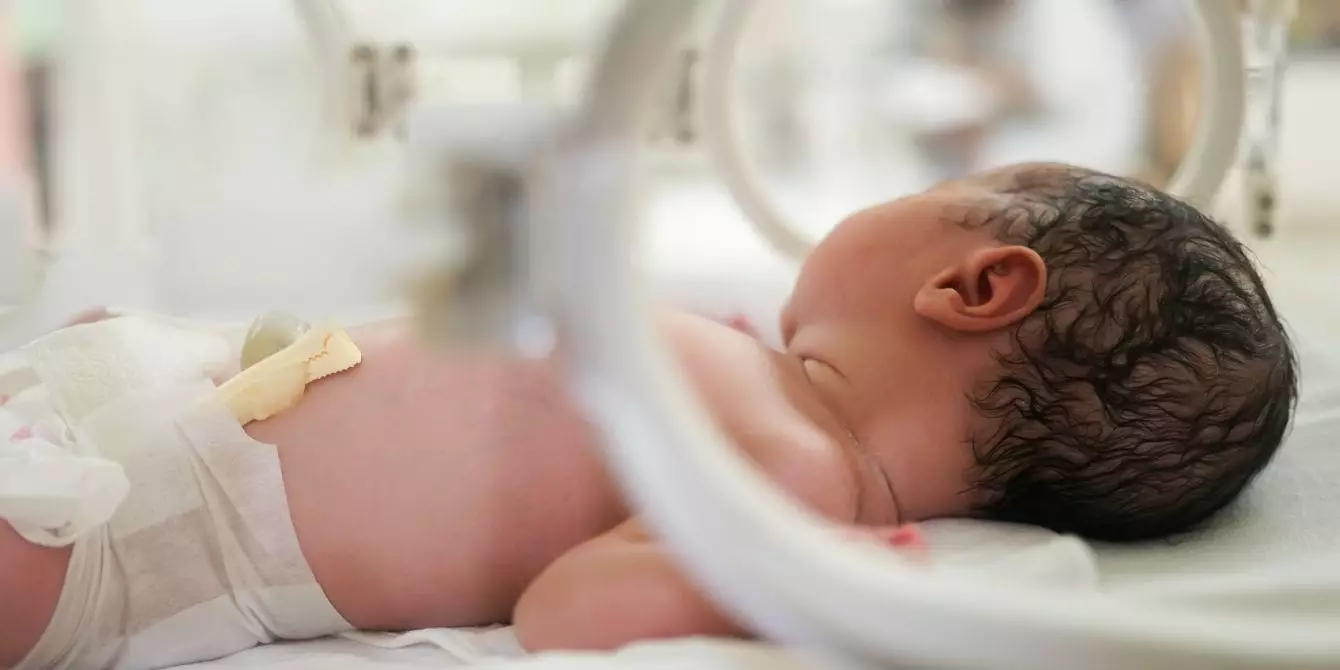
Preterm birth, which affects approximately 10% of pregnancies, is not merely a statistical concern. The growing evidence of its detrimental effects on both physical and developmental health is alarming, with preterm infants facing heightened risks for conditions such as respiratory illnesses, long-term developmental delays, and increased mortality rates. Given that certain populations, particularly Black and Native American women, have higher rates of preterm births, the failure of Makena poses significant questions regarding equitable healthcare access and outcomes.
The withdrawal of Makena from the market leaves many vulnerable groups with fewer options for prevention. The announcement that approximately 350,000 women have utilized Makena over the years highlights the reliance on this medication among at-risk populations. For these women, the absence of an effective pharmaceutical option could signify a stagnation in progress towards better maternal and infant health, particularly in socioeconomically disadvantaged groups.
While alternatives like compounded injectable progesterone may still exist, the concerns raised by recent study findings could deter healthcare providers from prescribing it. This hesitation underscores the persistent gap in understanding and addressing the complexities of preterm birth prevention. Furthermore, critics crow about the potential for deepening health disparities, particularly for marginalized populations who remain the most affected by preterm birth.
The debate surrounding Makena’s withdrawal reflects broader systemic issues in healthcare, especially pertaining to race and socioeconomic status. Organizations like the NAACP have rightfully pointed out that taking away Makena can exacerbate health inequalities among Black women, who are statistically more vulnerable to preterm births. The medical community must grapple with this reality and confront the intersectionality of maternal health, race, and healthcare access.
Moving forward, it is essential for researchers and policymakers to focus on comprehensive studies that encompass a variety of populations, particularly those most impacted by preterm birth. Establishing rigorous, well-designed studies that can both validate and challenge existing beliefs about maternal health interventions will be critical. Engaging disadvantaged communities in this process not only fosters trust but also ensures that their specific needs and challenges are adequately addressed.
The withdrawal of Makena should serve as a rallying point for advocates of maternal health reform. The emphasis should be on promoting transparency in pharmaceutical research and ensuring that interventions maintain rigorous efficacy standards. Only through a commitment to equitable healthcare practices and extensive research can future interventions hope to match the aspirations and needs of all expectant mothers, particularly those facing the anxieties of preterm birth. The complexity of this challenge demands a unified effort and unwavering dedication to dismantling barriers within maternal health, ultimately working toward a future where all pregnancies can thrive without the looming threat of premature delivery.